When Can I Drink Again After Breaking My Bone
- Inquiry article
- Open Admission
- Published:
Alcohol exposure decreases osteopontin expression during fracture healing and osteopontin-mediated mesenchymal stalk jail cell migration in vitro
Journal of Orthopaedic Surgery and Enquiry volume 13, Commodity number:101 (2018) Cite this commodity
Abstract
Background
Alcohol consumption is a risk factor for impaired fracture healing, though the mechanism(s) past which this occurs are not well understood. Our laboratory has previously shown that episodic alcohol exposure of rodents negatively affects fracture callus development, callus biomechanics, and cellular signaling which regulates stalk cell differentiation. Here, we examine whether alcohol alters chemokine expression and/or signaling activity in the mouse fracture callus during early fracture healing.
Methods
A mouse model for booze-impaired tibia fracture healing was utilized. Early fracture callus was examined for alcohol-furnishings on tissue composition, expression of chemokines involved in MSC migration to the fracture site, and biomechanics. The effects of booze on MSC migration and prison cell adhesion receptors were examined in an in vitro system.
Results
Mice exposed to alcohol showed decreased evidence of external callus formation, decreased callus-related osteopontin (OPN) expression levels, and decreased biomechanical stiffness. Alcohol exposure decreased rOPN-mediated MSC migration and integrin β1 receptor expression in vitro.
Conclusions
The furnishings of alcohol exposure demonstrated here on fracture callus-associated OPN expression, rOPN-mediated MSC migration in vitro, and MSC integrin β1 receptor expression in vitro have non been previously reported. Understanding the effects of booze exposure on the early on stages of fracture repair may allow timely initiation of treatment to mitigate the long-term complications of delayed healing and/or fracture non-union.
Background
While well-nigh patients suffering a bone fracture savour an unproblematic recovery, impaired fracture healing [delayed union, not-spousal relationship] occurs in approximately 5–10% of patients [1], with upwards to 19% of patients with open tibial shaft fractures progressing to not-spousal relationship. [2]. There are several factors that contribute to impaired fracture healing, one of which is excessive alcohol consumption [3,4,five,six]. Patients with non-unions have increased morbidity [7] and often require farther surgical interventions, which have limited efficacy and are costly to the healthcare arrangement. Understanding the biology of alcohol-impaired fracture healing may lead to the development of non-surgical strategies to forestall or reverse the process.
Alcohol consumption affects bone remodeling [3, 8], and rodent studies have documented the deleterious effects of chronic booze administration on fracture healing [9,ten,eleven]. Our laboratory has demonstrated that episodic alcohol exposure negatively affects both bone remodeling and the healing of experimentally induced fractures in rodents, and appears to specifically bear upon cartilaginous callus formation [8, 12,thirteen,14,fifteen,xvi,17,xviii,19,twenty,21,22,23]. Cartilaginous callus formation depends on the presence and action of mesenchymal stalk cells (MSC) at the fracture site. Wezeman and colleagues [24, 25] demonstrated that alcohol exposure inhibited the in vitro osteogenic differentiation potential of primary cultured human MSC. Stem cells take the power to drift following injury, and piece of work shows that MSC abode to the site of a healing fracture [26,27,28,29]. While the exact part of these migrating cells in fracture healing has not been determined, two chemokines, stromal prison cell-derived gene-i (SDF-1α) [30], and osteopontin (OPN) [31] induce MSC homing following injury. Reports suggest that OPN, specifically through interaction with the integrin β1 receptor, may regulate MSC migration [24, 32]. The furnishings of alcohol on MSC migration following fracture accept not been examined nor has any work examined the effects of booze on OPN-related signaling activity post-obit fracture.
Our laboratory has demonstrated that the localization of exogenously delivered MSC to the fracture site may differ between command and booze-exposed mice [20]. We hypothesized that i potential mechanism underlying the inhibition of cartilaginous callus germination observed in alcohol-exposed rodents could be related to a disruption of SDF-1 and/or OPN expression in fracture callus of animals exposed to alcohol. We farther hypothesized that perturbations of fracture site-associated chemokine expression in alcohol-exposed animals would be associated with changes in fracture callus tissue composition and structural backdrop. In an effort to link alcohol exposure to MSC activity, we utilized an in vitro system to test the hypothesis that alcohol treatment attenuates the migration of main cultured rodent MSC.
Methods
This study investigates the effects of alcohol exposure on the early on stages of fracture healing using a mouse model of tibia fracture. This written report received approval in 2012 from the Loyola University Chicago, Institutional Animal Intendance and Apply Commission (IACUC #12–057). Sixty-6 wild type (C57BL/6) male person mice aged 6–seven weeks were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were acclimated for 1 week in our fauna care facility prior to initiation of the experiment and were randomly assigned to either the saline control or booze exposure handling groups.
Alcohol exposure
Mice received either intraperitoneal (IP) injections of 20% (five/v) ethanol/sterile isotonic saline solution made from 100% molecular grade ethanol (Sigma-Aldrich, St. Louis, MO) at a dose of 2 k/kg, or sterile isotonic saline at like volumes. The alcohol exposure regimen was one time daily IP injections given for 3 days ane week prior to fracture, and so again, the 3 days leading up to fracture (4 days between injection cycles). Using this dosing regimen, a blood booze level (BAL) of ~ 200 mg/dl was achieved one h post injection, (at the time of fracture injury) to mimic the heavy episodic drinking patterns observed in intoxicated trauma patients [33]. Booze administration was connected during the post-fracture period to mimic patient post-trauma alcohol consumption patterns [16, 34].
Fracture surgery protocol
Mouse tibia fractures were created every bit previously described [18]. Briefly, anesthesia was induced with a combination of intraperitoneal ketamine (0.75 mg/kg) and xyalzine (0.08 mg/kg). Animals were prepped for sterile surgery, given prophylactic gentamicin (5 mg/kg) and anesthetized with inhaled isoflurane. An incision was made over the left proximal tibia, skin was retracted proximally to betrayal the patellar tendon, and a 27 M needle was used to gain access to the tibia intramedullary canal from a lateral parapatellar position. A stainless pivot (0.25 mm, Fine Science Tools, Foster City, CA) was inserted into the tibial culvert to stabilize the bone. The incision was retracted distally to overlie the mid tibial diaphysis and angled os scissors were used to create a mid-shaft transverse fracture. The pin was cutting affluent with the proximal tibia and the wound was sutured. Mice were given i cc of saline subcutaneously for resuscitation. All mice received 3 doses of buprenorphrine (0.05 mg/kg) subcutaneously for hurting command q8 hours mail-operatively. Past 24 h mail-operatively, mice were agile and weight bearing on the injured limb.
Specimen processing
Fractured and contralateral tibiae were harvested from mice following euthanasia at iii or vii days postal service-fracture. Fracture callus specimens harvested at 3 days post-fracture were utilized for either histology or chemokine protein expression assay. Fragility of callus specimens at iii days post-fracture did not allow for biomechanical testing or Micro-CT analysis at this time signal, and then callus specimens harvested at 7 days post-fracture were utilized for biomechanical, Micro-CT likewise as chemokine analysis. Care was taken to dissect all visible soft tissue from the callus of the fractured limb. Tibiae taken for biomechanical testing were wrapped in saline soaked gauze and stored at − twenty °C. Samples for histology or micro CT testing were placed into 10% neutral buffered formalin and stored at room temperature. Samples taken for protein analysis were snap frozen in liquid nitrogen and stored at − eighty °C.
Gross morphology and histology
Photographs of gross morphology were taken of tibiae prior to biomechanical testing (Fig. 1). For histology, specimens were fixed in 10% formalin for a minimum of 7 days and and then decalcified in 10% EDTA with agitation for vii days. Sagittal sections were stained with H&E and were mounted on glass slides.
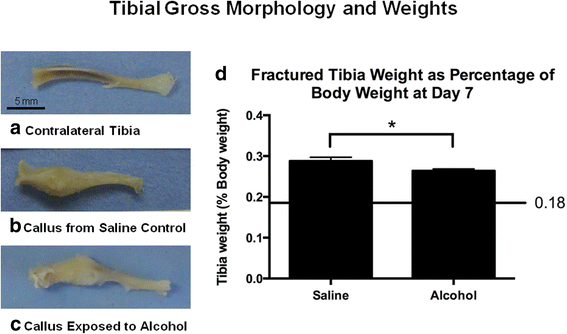
Tibia fracture morphology and weights. a Contralateral intact tibia from a saline control mouse. b Fracture callus in situ at seven days postal service-fracture from a saline control mouse. c Fracture callus in situ at seven days post-fracture from an booze-exposed mouse. The calluses from the saline control and alcohol-exposed mice were similar in size, just the alcohol-exposed callus was less robust appearing. Scale bar in a represents 5 mm and applies to b and c besides. d Tibial weight at vii days mail service-fracture equally a per centum of the mouse total trunk weight (tBW). The line represents intact contralateral limbs, which were 0.xviii ± 0.01% tBW for both saline command and alcohol-exposed mice. Data are shown as hateful ± SEM, n = 9/group. *p = 0.03 by Student's t examination
Sample training and poly peptide assay
Samples were removed from − lxxx °C and were placed on dry out ice. Whole tibia, whether fractured or intact contralateral, were weighed. Fracture callus was isolated from injured tibiae using a Dremel rotary cutting tool (Dremel, Racine, WI); contralateral intact tibiae were left undisturbed. A Spex Freezer Mill (SPEX, 6770 SamplePrep, Metuchen, NJ) was then used to pulverize the specimens while frozen in ane mL lysis buffer (from x mL RIPA Buffer, ane tablet Protease Inhibitor Cocktail, 100 μL Halt Phosphatase Inhibitor). Total protein in the samples was measured using the Pierce™ BCA assay (ThermoFisher Scientific, Rockford, IL). OPN and integrin β1 protein levels were measured via western blot. xv μg total protein per sample was resolved on a 4 to xx% SDS-PAGE gel, was transferred to a PVDF membrane, and was probed with either the Phosphoprotein 1 (SPP1 or Osteopontin ane) rabbit anti-mouse monoclonal antibody (Epitomics, Burlingame, CA) or the anti-integrin β1 rabbit polyclonal antibiotic (abcam, Cambridge, MA). To assess protein transfer, the membranes were stained with Coomassie blue [18] after total OPN detection (~ 33 kDa). Densitometric analyses were carried out using Epitome Lab software (Bio-Rad, Hercules, CA). Total OPN values were normalized to a ~ 40 kDa ring on the Coomassie stained membrane. SDF-1α was assayed using the mouse CXCL12/SDF-1α Quantikine ELISA (R&D Systems, Minneapolis, MN). R&D Systems Spike and Recovery protocol for validating untested samples was used to ostend exam validity (data not shown).
Biomechanical testing
Injured and contralateral tibiae, harvested from control and alcohol-exposed mice at seven days mail-fracture, were used for 4-indicate angle analysis. The contralateral tibias served as the uninjured control group. Samples were thawed at room temperature and were loaded into a customized 4-point bending apparatus (long-span altitude vii mm; short-bridge distance 3 mm) and tested at 0.5 mm/s using a biomaterial testing machine (Model 5544; Instron Corporation, County, MA). Calluses were centrally positioned within the short span. Load-deflection curves were obtained, and the slope of the linear portion was taken as the callus stiffness.
Micro-CT analysis
Specimens were placed into a tube containing 100 μL of formalin with a small wick of gauze at the base of operations. High-resolution phase contrast synchrotron μCT was performed with the Argonne National Laboratories Advanced Photon Source beamline ii-BM. Synchrotron μCT produces images with sharper features and stage contrast improves sensitivity to different soft tissue types [35], allowing easier/more authentic segmentation of soft tissue. Airplane pilot scans showed a 600-mm altitude betwixt the specimen and detector optimized contrast between air and soft tissue compared to other separations. Final imaging parameters were 24.3 keV, 600 mm imaging distance, × 2.v lens, 0.12° rotation between paradigm conquering with 300 ms exposure, and a (2 Yard)2 CCD. Reconstructions consisted of 2.8 μm isotropic voxels. Information was reconstructed using a customized in-house plan similar to ANKAphase [36] based on the Paganin single-distance phase retrieval algorithm [37]. To minimize option bias, specific parameters were established for selecting the portion of fracture callus to be analyzed. The distal terminate was set where the cross-sectional expanse was 3.9 mm2. A fixed length of ii.98 mm of callus proximal to the distal end was analyzed for each sample. Between the proximal and distal ends, at that place were 1065 slices. We measured callus book every 15th slice and interpolated the callus volumes between measurements. The total volume (TV) of the callus was defined every bit the volume of all voxels within the callus, was performed by manually outlining the border of each specimen to define the region of involvement. To quantify tissue composition in the ROI, 32 B, grayscale images were established by visual segmentation. Absolute numbers for the calculated volumes did not alter with slight shifts of the threshold values, and changes observed when the thresholds were varied were like among specimens. Bone volume (BV) was divers between 0.000691 and 0.00417 on the image histogram, mineralized tissue was from 0.0000619 to 0.000691, and soft tissue was defined as the remaining tissue within the ROI less than 0.0000619 on the epitome histogram. These thresholds were applied to each sample, and the volumes were calculated using the BoneJ plugin [38] for ImageJ. In addition to the volume, the polar moment of inertia (I political leader) was calculated using the slice geometry function in BoneJ. I pol values were averaged over the 71 sections as previously described [39]. The polar moment of inertia measures the distribution of mass in a cross section of a material, serving as a description of the geometry of the callus and is proportional to its resistance to bending.
In vitro MSC migration
Primary mice (C57BL/6) Mesenchymal Stem Cells (Invitrogen, Carlsbad, CA) were used for the migration analysis. Cells were used for all experiments at Passage 9. MSC were added to a growth medium consisting of DMEM/F-12 medium with GlutaMAX™-I, 10% MSC-Qualified FBS, and 5 μg/mL gentamycin. Cells were and then incubated at 37 °C in 5% COii on flasks seeded at 5000 cells/cm2 until plates were ~ 90% confluent. MSC were discrete using a TrypLE solution (Life Technologies, Grand Island, NY), were washed twice with sterile PBS, and then were resuspended in medium (DMEM + 0.1% BSA) at a concentration of xxx,000 cells per 0.04 mL. The in vitro cell migration analysis was carried out using ChemoTx® disposable chemotaxis system 96-well plates with 8-μm pore size (NeuroProbe, Gaithersburg, Doc). Upper wells were loaded with 30,000 MSC suspended in medium. Medium with recombinant murine OPN (R&D Systems) at concentrations of ane and 5 μg/ml was added to the lower wells. Medium solitary was used equally the negative control. Later 24 h of incubation, cells remaining on the summit surface of the membrane were removed. Cells migrating to the bottom surface of the membrane were fixed using 2.five% glutaraldehyde, stained with hematoxylin, and counted under a lite microscope. Each assay condition was carried out in triplicate, and the average value was reported. The analysis was repeated four times with different MSC cultures. The assay conditions tested were (1) MSC cultured with 50 mM ethanol, no ethanol during migration assay (2) Ethanol nowadays only during migration (50 mM ethanol added to the lower wells), and (three) 24 h of MSC cultured in the presence of l mM ethanol and ethanol added to lower assay well. 50 mM ethanol is equivalent 230 mg/dL, equivalent to the BAL of mice at the time of fracture surgery.
MSC isolation
Mesenchymal stem cells were isolated from 6 to 7-calendar week-old male Lewis rats using a modified protocol as described previously [twoscore, 41]. Briefly, animals were humanely euthanized, and both tibiae and femurs were harvested. The proximal and distal ends of each bone were sheared off with bone snips. The marrow of each bone was flushed with D-MEM supplemented with 20% FBS, and the resulting marrow jail cell interruption was filtered through a seventy-μM filter to remove any contaminating bone or cell clumps. This cell intermission was centrifuged at 450 g for 5 min; the pellet was resuspended in 5 mL of D-MEM containing 20% FBS and transferred to a T-25 cmtwo culture flask. The civilisation medium was carefully replaced later on 24 h of culture and and then every three–4 days as needed to retain plastic-adherent cells and to remove whatsoever contaminating non-adherent cell populations. The timing of the replacements of culture media following culture initiation to remove contaminating cell populations from the primary MSCs differs from protocols for the isolation of other related stem cell populations such as those from developed muscle tissue, in which media changes are not performed until later (5 days) when plastic adherent cells of myogenic origin are established [42]. The MSCs were sub-cultured before colonies became multilayered. Later i passage for expansion, the cells were then harvested and aliquoted at 1 million cells/mL in freezing medium (DMEM supplemented with 20% FBS and ten% DMSO) and deposited in liquid nitrogen vapor phase storage.
Integrin beta1 expression
Rat MSCs were cultured in depression glucose, GlutaMAX™ D-MEM (Gibco, ThermoFisher Scientific, Rockford, IL) supplemented with 10% FBS (Gibco, ThermoFisher Scientific). Cells were grown in 75 cm2 culture flasks until approximately 80% confluent. Cells were then exposed to media lone or media plus 50 mM EtOH for 24 h with EtOH loss past evaporation mitigated by culturing in a sealed organization with excess EtOH at the aforementioned concentration as the treatment. Integrin β1 mRNA and protein expression were measured using qRT-PCR and western blotting, respectively. For both, cells were harvested using TrypLE Express (1×, ThermoFisher) and pelleted by centrifugation. So, either total RNA was isolated using the Qiagen RNeasy Mini Kit (Qiagen, Carol Stream, IL) or total protein was isolated using 1 mL Lysis Buffer (from 10 mL RIPA Buffer, one tablet Protease Inhibitor Cocktail, 100 μL Halt Phosphatase Inhibitor). RNA was quantified using a NanoDrop ND-thou spectrophotometer and quality assessed with the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). RNA was used to create a cDNA library (High-Capacity cDNA Reverse Transcription Kit, ThermoFisher). cDNA libraries were subjected to quantitative real-time PCR analysis (Practical Biosystems 7500 Fast qRT-PCR). The resulting data were analyzed by the delta-delta Ct method. TaqMan Fast Advanced Primary Mix, uniform, and TaqMan FAM primer probes specific for integrin beta1 and beta2 microglobulin (β2M), the endogenous control were used (ThermoFisher). Integrin β1 poly peptide expression was assayed using western blot equally previously described above in Sample Preparation and Protein Analysis.
Data analyses
Data are expressed as mean ± SEM. Statistical assay was performed using Prism v6.0a (GraphPad Software, La Jolla, CA). Educatee's t test was used to compare saline control and alcohol-exposed groups for tibial weight, μCT tissue composition, and bending stiffness. Chemokine protein expression levels were analyzed by ii-style ANOVA using injury condition (intact or fracture) and treatment (saline or alcohol) as factors with Tukey'southward post-hoc testing. Cell migration data were analyzed past 1-manner ANOVA using pre-defined comparisons with Holm-Sidak post hoc testing. Ix pairwise comparisons were performed to examine the furnishings of OPN dose and alcohol exposure (see Fig. 7 legend). Protein and mRNA levels of integrin β1 were compared with Student's t examination. A p value < 0.05 was considered significant.
Results
Effects of alcohol on fracture callus morphology and structure
No significant effects of alcohol handling on mouse body weight at the time of euthanasia were noted (data not shown). Figure i shows representative tibia samples from an uninjured saline command mouse (Fig. 1a), a fracture-injured saline control (Fig. 1b), and a fracture-injured alcohol-exposed animal (Fig. 1c) at 7 days post-injury. Effigy 1d shows the tibial weight of the fractured tibia normalized to total mouse body weight (BW). Fractured tibia from mice in the alcohol-exposed group weighed significantly less (p = 0.03) compared to fractured tibia from saline command animals.
We have shown that episodic booze treatment of mice inhibits cartilaginous external fracture callus germination at post fracture days 6 and 9 [43]. Hither, we examined H&Eastward-stained sections of the fracture site in saline and booze-treated mice at day 3 post-injury for show of alcohol-related effects on early mail service-fracture granulation tissue accumulation (Fig. ii). The fracture site from saline control animals shows aggregating of granulation tissue (Fig. 2a, boxed areas) and early cartilage formation (arrow). In contrast, the fracture site of booze-exposed animals shows virtually no accumulation of either granulation tissue (Fig. 2b, boxed area) or cartilage formation. Samples shown in Fig. 2 are representative for each treatment grouping.
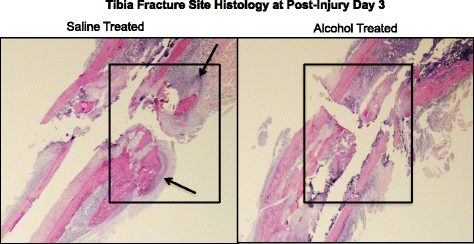
Fracture callus histology (H&E staining x×). Histological structure of the fracture site is shown at 3 days post-fracture in saline command (a) and alcohol exposed (b). The fracture site of saline control mice shows testify of granulation tissue accumulation (boxed area) and the presence of early cartilaginous callus germination (arrows). In dissimilarity, the injury site of alcohol-exposed mice shows no evidence of granulation tissue accumulation or ormation or cartilage tissue. n = ii per group
Effects of alcohol on fracture callus biomechanics
Fractured tibia specimens were tested at 7-days mail service-injury for biomechanical maximum load to failure and bending stiffness using 4-point bending. A big plastic deformation of the fractured tibia specimens was observed while testing, causing the specimen to wedge in the 4-point testing appliance, making load to failure measurements unreliable at this time point (data not shown). Callus stiffness was measurable at 7 days post-injury by 4-point bending and was significantly decreased in calluses from alcohol-exposed mice compared to saline controls (Fig. three).
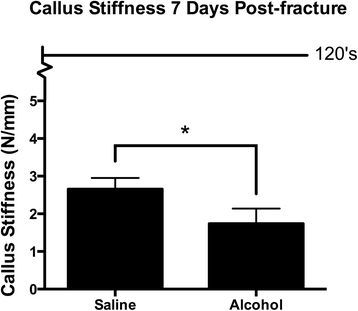
Fracture callus biomechanical analysis. Biomechanical stiffness of post-injury day 7 fracture callus from saline control and alcohol-exposed mice was assessed using a 4-point bending appliance. The line in the graph represents stiffness of intact contralateral limbs, which were 122 ± 12 and 127 ± thirteen N/mm for saline control and alcohol-exposed mice, respectively. Calluses from the alcohol-exposed mice were significantly less stiff than the saline controls. Data are shown as mean ± SEM, due north = ix/group. *p = 0.04 by Educatee'due south t examination
Effects of alcohol on fracture callus microstructure
Fracture callus specimens from the saline control and alcohol-exposed groups were imaged with phase contrast synchrotron μCT at 7 days mail service-injury to determine full callus volume (Television receiver) and the percent of callus composed of soft tissue and mineralized tissue. Figure 4a, b shows representative calluses from the saline control and alcohol-exposed groups, respectively. Within the callus, the white tissue is mature, pre-existing os, while the black tissue is mineralized tissue formed since fracture. Greyness tissue is soft tissue (based on segmentation as described in the "Methods" department). Total callus volume was non significantly unlike between the experimental groups, measuring 19.83 ± 0.85 and 21.29 ± 1.29 mmthree for the saline control and alcohol-exposed groups, respectively (information not shown). The pct soft tissue book of the callus was not significantly different betwixt the experimental groups, (Fig. 4c, left most bars). Full newly mineralized tissue formed since fracture in the callus (callus tissue within the medullary canal and external to the os shaft) trended toward a significant deviation (p = 0.08) for the saline control and booze exposed, respectively (Fig. 4c, middle bars). When selecting only the external compartment of the callus, in that location was a significant divergence (p = 0.03) seen in newly mineralized tissue (Fig. 4c, right bars). The total volume (in percentage) of newly mineralized tissue in the saline command callus was 17.8 ± i.5, and 13.0 ± 1.0 for the alcohol-exposed group, a 27% decrease. The average polar moment of inertia (I politician) for the calluses was not significantly different between groups.
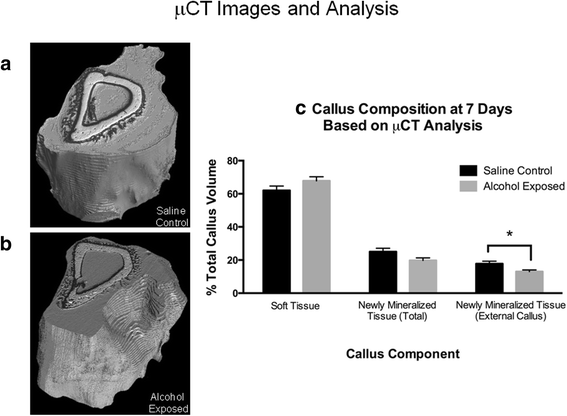
Micro-CT assay of fracture callus at day vii mail-fracture. Representative 3D reconstructions of a saline command and b alcohol-exposed fracture calluses. The white area is mature, pre-existing bone; blackness area is mineralized tissue formed since fracture; gray expanse is soft tissue (based on sectionalisation as described in the "Methods" section). There is more newly mineralized tissue seen in the saline control callus than in callus from booze-treated mice. c Quantification of soft tissue, full newly mineralized tissue, and newly mineralized tissue in the external callus as a percentage of the full callus book (%Telly). In that location is significantly less newly mineralized tissue in the external callus from alcohol-exposed mice compared to saline controls. Data are shown as mean ± SEM, n = 5/group. *p = 0.03 past Student'due south t test
Effects of booze on fracture callus OPN and SDF-1α protein levels
OPN protein expression was examined by western blot analysis in fracture callus samples from saline control and alcohol-exposed animals. The uninjured, contralateral saline control tibiae were used to scale OPN protein levels for semi-quantitative analysis. OPN was significantly decreased (p < 0.05) in fracture callus, regardless of treatment at day 3 postal service-fracture compared to the contralateral uninjured limbs (Fig. 5a). At twenty-four hour period 7 post-fracture, OPN protein levels were significantly increased (p < 0.05) in the fracture callus of the saline control compared with contralateral uninjured limbs (Fig. 5b). This increment in fracture callus-associated OPN expression at day seven post-injury was significantly blunted (p < 0.05) in booze-exposed mice. SDF-1α was assayed in callus samples by sandwich ELISA, and values were normalized to microgram of total protein. At both 3 and 7 days post-fracture SDF-1α expression was significantly decreased (p < 0.05) in fracture calluses compared to the contralateral uninjured limbs (Fig. 6a, b). There was no upshot of alcohol exposure on SDF-1α expression in fracture callus tissue at mail service-injury days 3 or vii.
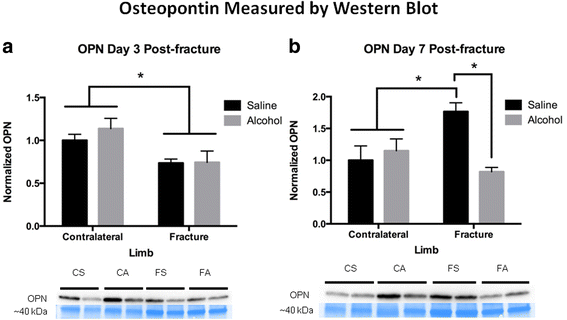
Osteopontin-i protein levels in fracture callus at a 3 and b vii days post-fracture. Bar graphs show fracture callus OPN levels. Below the graphs are representative western blots for the respective treatment groups and time points. For western blots, CS = contralateral tibia saline control group, CA = contralateral tibia alcohol-exposed group, FS = fracture callus from saline control group, and FA = fracture callus from alcohol-exposed group. Bar graph are shown as hateful ± SEM, n = 3–4/group for contralateral, and eight–9/group for fracture callus. *p < 0.05 by ane-way ANOVA with Tukey's mail hoc test
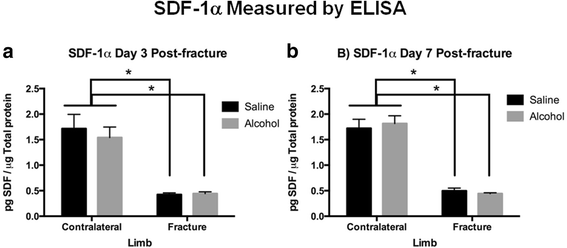
SDF-1α Protein Levels in Fracture Callus at a 3 days and b 7 days Post-Fracture. Bar graphs describe levels of SDF-1α in fracture callus or intact tibia specimens every bit pg SDF-1α per μg of total protein in the sample every bit measured by ELISA. Contralateral = intact not-fractured tibia, Fracture = callus from fractured tibia, Booze = episodic booze exposure, and Saline = control mice. Data shown as mean ± SEM, n = 3/group for contralateral, and 9/group for fracture callus. *p < 0.05 by one-mode ANOVA with Tukey'due south postal service hoc test
Effects of alcohol on in vitro MSC migration and integrin β1 expression
Prior research has shown that OPN acts as a chemokine to facilitate MSC migration via the integrin β1 receptor [32, 44]. We examined whether booze exposure would affect OPN-mediated MSC migration in vitro. First, we demonstrated that primary cultured mouse MSC migrated toward rOPN (5 or 1 μg/ml) in a dose-dependent manner, with negligible migration observed in cells not stimulated with rOPN (Fig. 7a). Primary MSC were then cultured in the presence of fifty mM ethanol for either 24 h prior to the analysis (pre-exposure), during the assay (concurrent exposure), or both. In each exposure regimen, MSC demonstrated significantly less migration toward 5 μg/ml rOPN. (Fig. 7a). A trend toward decreased MSC migration was observed in cells exposed to l mM ethanol and stimulated with 1 μg/ml rOPN (Fig. 7a). The chemotactic alphabetize, expressed every bit fold change in MSC migration over unstimulated control MSC migration is shown in Fig. 7b.
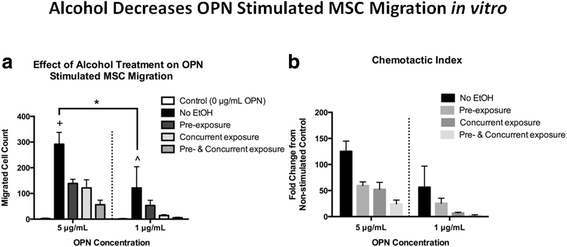
Effect of alcohol on in vitro MSC migration. Bar graphs show a migration of principal MSCs every bit a part of rOPN concentration and alcohol handling and b chemotactic index of MSC migration data. Control = MSC migration in the absence of rOPN, no EtOH = rOPN stimulated MSC migration with no exposure to booze, pre-exposure = 24 h. pre-incubation of MSC in l mM EtOH, concurrent exposure = MSC migration assay performed in the presence of 50 mM EtOH in the rOPN well, pre- and concurrent exposure = combination of pre-exposure and concurrent exposure treatments. Data are shown every bit hateful ± SEM, northward = 4 experiments/group, each performed in triplicate. A two-way ANOVA with Holm-Sidak post hoc testing was performed on pre-defined comparisons of the no EtOH grouping versus every other bar at either the 5 or 1 μg/mL rOPN concentration and comparing no EtOH at the five and one μg/mL rOPN concentrations. *p < 0.05 comparing no EtOH at the v and 1 μg/mL rOPN concentrations. +p < 0.05 comparison no EtOH to all other bars in the 5 μg/mL rOPN group (to left of dashed line). All conditions showed statistically less MSC migration than the no EtOH grouping. ^p < 0.05 comparing no EtOH to all other bars in the 1 μg/mL rOPN group (to right of dashed line). All conditions except pre-exposure showed statistically less MSC migration than the no EtOH group. Each experiment was repeated at least iii times utilizing unique primary MSC cultures
In an attempt to determine why ethanol exposure caused a decreased migration of primary MSC toward rOPN, nosotros examined integrin β1 expression in primary MSC cultured in the presence of fifty mM ethanol for 24 h. We institute that both mRNA (Fig. 8a) and protein levels (Fig. 8b) for the integrin β1 receptor were significantly decreased in MSC exposed to 50 mM ethanol in vitro (p = 0.002 and 0.003, respectively). In contrast, ethanol exposure did not significantly change the expression of CD44 (another OPN receptor) in cultured MSC (information not shown).
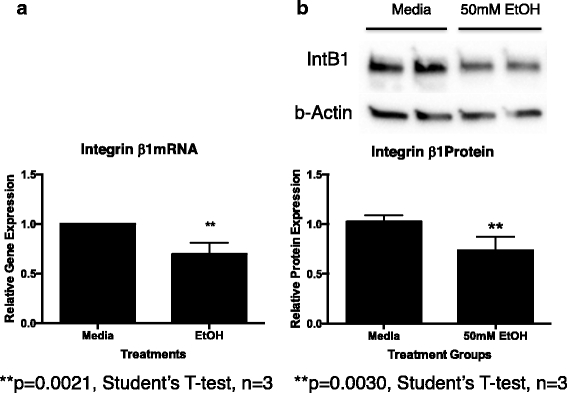
Effect of alcohol on principal cultured MSC integrin β1 mRNA and protein expression. Primary rat MSC were cultured in media lone or media plus l mM ethanol for 24 h. Cells were harvested and used for a mRNA or b total poly peptide isolation as described. Int β1 mRNA levels were assessed by qRT-PCR every bit described. Int β1 poly peptide levels were assessed by western blot analysis as described. a mRNA: media vs EtOH p = 0.0021. b Poly peptide: media vs EtOH p = 0.0030. Each experiment was repeated at least three times utilizing unique main MSC cultures. *p < 0.05 by Student's t exam
Discussion
In this study, we examined the effects of episodic alcohol exposure on the early stages of fracture healing in a model system of mouse tibia fracture. We show testify that the accumulation of granulation tissue and mineralization of the external cartilaginous callus forming at the fracture site are negatively affected by alcohol exposure. We also demonstrated decreased fracture callus biomechanical stiffness at twenty-four hours vii post-injury. Because MSC localization to the fracture site is critical to external callus germination, nosotros examined the furnishings of alcohol on chemokine expression in the early callus and showed significantly decreased levels of OPN in callus from alcohol-exposed mice at 7 days post-injury. Finally, we demonstrated that alcohol exposure decreases MSC integrin β1 receptor expression and blunts osteopontin-induced MSC migration in vitro. Taken together, these results suggest that alcohol-related inhibition of fracture callus formation may exist related in part to its perturbation of OPN-mediated MSC localization to, or activity at, the fracture site. Our observations that booze exposure decreases OPN expression during early bone fracture healing and that exposure of cultured MSC to booze alters integrin β1 receptor levels and inhibits the migration of stalk cells toward rOPN take not been previously reported. Although our episodic alcohol regimen likely causes a delay in the fracture repair rather than a non-healing fracture, the effects of alcohol observed on early fracture repair may have important repercussions whether the concluding upshot is delayed healing or non-marriage [45].
The effects of alcohol nosotros observed on fracture callus histology, microstructure, and biomechanical parameters provide evidence that booze negatively affects the early fracture healing process. Our histological data demonstrates a qualitative effect of pre-and post-injury episodic booze exposure on early on accumulation of granulation tissue at the fracture site. The observations reported hither on the furnishings of pre- and post-fracture booze exposure are in line with our previously published data on pre-injury alcohol exposure and callus formation [18, nineteen, 43]. Further, while total volume of the callus did not differ betwixt groups (as measured by μCT), the per centum of newly mineralized tissue was significantly lower in alcohol-exposed animals than in saline controls. We take previously shown decreased new bone volume at the callus in response to episodic booze exposure at 14 days mail service-injury [20]. Our current data demonstrates that this event on callus mineralization occurs as early on as seven days. Fracture callus from alcohol-treated mice was less stiff in 4-point bending than samples from corresponding saline-treated mice. Control callus stiffness values obtained in our study were similar to those at 7 days post fracture obtained by Hiltunen [46]. Thus, callus from saline control mice is of ameliorate quality than from episodic alcohol-exposed mice, reflected by the differences in cartilaginous callus observed histologically [43] and newly mineralized tissue measured via μCT. Stiffness of a callus is afflicted by tissue amount, composition, geometry, or a combination of factors. The decreased stiffness of calluses from booze-treated mice likely relates to a decreased percent of newly mineralized tissue every bit information technology is not due to changes in callus volume or distribution of the mass, as neither Telly nor I pol were affected by booze exposure.
Nosotros have previously demonstrated attenuation of Canonical Wnt signaling action at the fracture site in alcohol-treated mice [18, 19], suggesting that booze may disrupt signaling through a cellular pathway important for MSC differentiation [47] and subsequent fracture healing [32]. The data presented here advise that alcohol also affects cellular signaling important for MSC localization to the injury site. Both OPN and SDF-1α are chemokines expressed at the fracture site which have previously been shown to be involved in MSC migration [30,31,32, 44]. At 3 days post-injury, callus-specific expression of OPN and SDF-1α were not afflicted by alcohol exposure in our model. Notwithstanding, alcohol exposure decreased callus-associated OPN levels 7 days mail-injury compared to the normal increase seen in saline controls. This ascertainment suggests that an booze-specific perturbation of OPN-mediated chemokine signaling could underlie, at least in part, the deficits in cartilaginous callus germination we accept previously observed in alcohol-exposed animals [43] by affecting either MSC availability or activity at the site of injury. While these specific chemokine levels at the fracture site appear not to be perturbed past alcohol at 3 days post-injury, nosotros cannot rule out that other early chemokine-related signaling in MSC may be affected by alcohol during early fracture repair.
Determination
A contempo clinical study showed that serum OPN levels were increased at 7 days mail-injury in patients sustaining a long bone fracture [48]. OPN knockout mice show decreased callus volume, reduced callus biomechanical properties and increased callus mineralization as compared to wild-type mice [49]. This report suggests that OPN expression at the fracture site may exist related to callus book and biomechanical strength of the callus, which agrees with our data. The exact role of OPN in bone mineralization is not clear [fifty], and, given its multiplicity of known functions, it could have different functions at dissimilar times post-fracture. Alcohol has other known furnishings on signaling activity at the fracture site [18, 19, 51], which complicates any straight comparisons betwixt our study and those utilizing OPN knockout animals. OPN has other important roles in bone including the modulation of hydroxyapatite formation during os mineralization [50], and then any alcohol-related attenuation of OPN activity could have other effects on fracture healing unrelated to its chemokine-related activeness. Although we did non observe alcohol-specific effects on SDF-1α levels in the fracture callus in our study, a prior investigation demonstrated that SDF-1α directs MSC migration afterward fracture [30]. This study used a os-grafting model of bone repair and measured the fourth dimension course of SDF-1α RNA expression past qPCR. While we did not prove either increases in SDF-1α poly peptide levels following fracture or alcohol exposure-related effects on SDF-1α levels in the callus, we cannot dominion out that SDF-1α-dependent MSC migration to the fracture site could exist occurring in our model.
The biological relevance of MSC migration to the fracture site during fracture repair is non understood [52]. Osteopontin has several potential biologic functions during fracture healing, including participation in angiogenesis [48, 49], stalk cell recruitment [31, 32, 44], stem jail cell differentiation [53, 54], and mineralization [50]. Our hypothesis that OPN-stimulated MSC migration may be a target of alcohol exposure was examined hither in vitro, showing a dose-dependent migration of MSC toward OPN, and that exposure of MSC to alcohol blunts this response. To our knowledge, there are no other reports to date on the effects of alcohol exposure on MSC migration. Experiments are currently in progress to examine the effects of alcohol on the migration potential of patient-derived bone marrow MSC. Previous studies have shown that stem cells obtained from patients with alcohol-induced osteonecrosis of the femoral head show reduced ability to differentiate toward an osteogenic lineage compared with MSC obtained from patients with femoral neck fractures (55), suggesting that alcohol corruption may cause global changes in MSC function, leading to skeletal diseases such as osteonecrosis and fracture non-union.
Information technology has previously been shown that MSC migrate toward OPN via a CD44-mediated pathway stimulated past hypoxic osteocytes [31]. Of note, during the very early stages of fracture healing, impairment to local vasculature tin can render the fracture site hypoxic relative to surrounding tissues [55]. Other reports suggest that OPN-mediated MSC migration occurs via interaction with the integrin β1 receptor [32, 44]. Our data showed that OPN stimulates primary MSC migration in a dose-dependent style in vitro, and that booze inhibited this migration. Nosotros demonstrated that booze treatment of master MSC significantly decreased both mRNA and protein levels of integrin β1. This data may partially explain the mechanism underlying the ethanol-related decrease in OPN-mediated MSC migration shown in the in vitro organization. Coupled with data showing decreased OPN expression in callus tissue from alcohol-treated mice, the data suggests that OPN-related signaling is targeted by alcohol exposure during the early on repair menstruation.
Limitations to the current study include furnishings of booze intoxication on post fracture fauna activeness and the fracture fixation technique. The administration of alcohol at an exhilarant level could modulate ambulatory profiles of mice and differences in mail-injury activeness could alter biomechanical loading at the fracture site and subsequently affect fracture repair [56]. While nosotros did not monitor rodent activeness, alcohol administration was performed at the starting time of the light cycle, giving animals several hours to metabolize alcohol prior to the night cycle and the menstruum of greatest activeness for rodents. With respect to fixation, the tibial intramedullary pin technique [eighteen,19,twenty] allows the injured os ends to remain in close proximity during healing and provides reasonable stability. Appliances for the rigid fixation of rodent fractures are available [57] and would eliminate variables associated with fixation, but rigid fixation results in cortical bridging of the fracture via intramembranous bone formation without appreciable external callus formation. As we believe that inhibition of external callus formation may be the primary defect in alcohol-exposed rodents, the utilize of a rigid fixation device would non exist appropriate for the electric current studies. Mice were eliminated from the study if whatever evidence of pivot migration was found during fracture callus drove, which could result in inadequate fixation, ensuring that all specimens utilized were appropriately stabilized.
From a clinical perspective, the electric current report is express primarily by the fact that it is a laboratory animal study. However, our results could impact clinicians involved in the treatment of delayed fracture union/non-matrimony, because the study identifies a potential novel mechanism underlying booze-related delayed fracture healing (fracture-associated chemokine expression and MSC migration), which could be amenable to targeted stand up-alone therapies or every bit an offshoot to surgical procedures for non-union. This study also provides data regarding the role of alcohol corruption equally a modifiable risk gene in fracture healing, and data from this investigation may eventually lead to targeted pharmacologic or cell-based therapies that restore fracture healing in patients suffering from alcohol abuse disorders without the demand for surgery.
Abbreviations
- BAL:
-
Claret alcohol level
- BV:
-
Os book
- BW:
-
Body weight
- EtOH:
-
Ethanol
- IP:
-
Intraperitoneal
- I pol :
-
Polar moment of inertia
- MSC:
-
Mesenchymal stalk cells
- OPN:
-
Osteopontin
- SDF1-α:
-
Stromal jail cell-derived factor-1
- Tv:
-
Total volume
References
-
Bishop GB, Einhorn TA. Current and future clinical applications of os morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;316:721–vii.
-
Hak DJ. Management of aseptic tibial nonunion. J Am Acad Orthop Surg. 2011;199:563–73.
-
Chakkalakal DA. Alcohol-induced bone loss and scarce bone repair. Alcohol Clin Exp Res. 2005;2912:2077–90.
-
Duckworth AD, Bennet SJ, Aderinto J, Keating JF. Fixation of intracapsular fractures of the femoral neck in young patients: risk factors for failure. J Bone Joint Surg Br. 2011;936:811–half dozen.
-
Nyquist F, Berglund Thousand, Nilsson Be, Obrant KJ. Nature and healing of tibial shaft fractures in alcohol abusers. Alcohol Alcoholism (Oxford, Oxfordshire). 1997;321:91–5.
-
Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, Della Rocca GJ, Mehta S, McKinley T, Wang Z, Steen RG. Epidemiology of fracture nonunion in 18 human basic. JAMA Surg. 2016;15111:e162775.
-
Schottel PC, O'Connor DP, Brinker MR. Fourth dimension merchandise-off every bit a measure of health-related quality of life: long bone nonunions accept a devastating bear on. J Bone Articulation Surg Am. 2015;9717:1406–10.
-
Jung MK, Callaci JJ, Lauing KL, Otis JS, Radek KA, Jones MK, Kovacs EJ. Alcohol exposure and mechanisms of tissue injury and repair. Alcohol Clin Exp Res. 2011;353:392–ix.
-
Elmali N, Ertem K, Ozen S, Inan Thou, Baysal T, Guner G, Bora A. Fracture healing and os mass in rats fed on liquid nutrition containing ethanol. Alcohol Clin Exp Res. 2002;264:509–13.
-
Nyquist F, Halvorsen V, Madsen JE, Nordsletten L, Obrant KJ. Ethanol and its effects on fracture healing and os mass in male rats. Acta Orthop Scand. 1999;702:212–half dozen.
-
Perrien DS, Wahl EC, Hogue WR, Feige U, Aronson J, Ronis MJ, Badger TM, Lumpkin CK Jr. IL-1 and TNF antagonists foreclose inhibition of fracture healing past ethanol in rats. Toxicol Sci. 2004;822:656–threescore.
-
Callaci JJ, Himes R, Lauing K, Roper P. Long-term modulations in the vertebral transcriptome of boyish-phase rats exposed to binge booze. Booze Alcoholism (Oxford, Oxfordshire). 2010;454:332–46.
-
Callaci JJ, Himes R, Lauing K, Wezeman FH, Brownson K. Binge booze-induced bone damage is accompanied by differential expression of bone remodeling-related genes in rat vertebral os. Calcif Tissue Int. 2009;846:474–84.
-
Callaci JJ, Juknelis D, Patwardhan A, Sartori M, Frost North, Wezeman FH. The furnishings of binge booze exposure on os resorption and biomechanical and structural backdrop are offset by concurrent bisphosphonate treatment. Booze Clin Exp Res. 2004;281:182–91.
-
Callaci JJ, Juknelis D, Patwardhan A, Wezeman FH. Binge alcohol handling increases vertebral bone loss following ovariectomy: compensation past intermittent parathyroid hormone. Alcohol Clin Exp Res. 2006;304:665–72.
-
Halcomb Eastward, Daly J, Davidson P, Elliott D, Griffiths R. Life beyond severe traumatic injury: an integrative review of the literature. Aust Crit Intendance. 2005;181:17–eight. xx-14
-
Lauing K, Himes R, Rachwalski M, Strotman P, Callaci JJ. Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in os loss and incomplete recovery of bone mass and strength. Booze (Fayetteville, NY). 2008;428:649–56.
-
Lauing KL, Roper PM, Nauer RK, Callaci JJ. Acute alcohol exposure impairs fracture healing and deregulates beta-catenin signaling in the fracture callus. Alcohol Clin Exp Res. 2012;3612:2095–103.
-
Lauing KL, Sundaramurthy S, Nauer RK, Callaci JJ. Exogenous activation of Wnt/beta-catenin signaling attenuates binge booze-induced deficient bone fracture healing. Alcohol Alcoholism (Oxford, Oxfordshire). 2014;494:399–408.
-
Obermeyer TS, Yonick D, Lauing 1000, Stock SR, Nauer R, Strotman P, Shankar R, Gamelli R, Stover Chiliad, Callaci JJ. Mesenchymal stalk cells facilitate fracture repair in an alcohol-induced impaired healing model. J Orthop Trauma. 2012;2612:712–8.
-
Sears BW, Volkmer D, Yong South, Himes RD, Lauing G, Morgan Thou, Stover MD, Callaci JJ. Rampage booze exposure modulates rodent expression of biomarkers of the immunoinflammatory response to orthopaedic trauma. J Bone Joint Surg Am. 2011;938:739–49.
-
Volkmer DL, Sears B, Lauing KL, Nauer RK, Roper PM, Yong South, Stover M, Callaci JJ. Antioxidant therapy attenuates deficient bone fracture repair associated with binge booze exposure. J Orthop Trauma. 2011;258:516–21.
-
Wezeman FH, Juknelis D, Himes R, Callaci JJ. Vitamin D and ibandronate forbid cancellous os loss associated with binge alcohol treatment in male rats. Bone. 2007;414:639–45.
-
Gong Z, Wezeman FH. Inhibitory effect of alcohol on osteogenic differentiation in human bone marrow-derived mesenchymal stem cells. Alcohol Clin Exp Res. 2004;283:468–79.
-
Wezeman FH, Gong Z. Adipogenic effect of alcohol on human bone marrow-derived mesenchymal stem cells. Alcohol Clin Exp Res. 2004;287:1091–101.
-
Devine MJ, Mierisch CM, Jang Due east, Anderson PC, Balian Grand. Transplanted bone marrow cells localize to fracture callus in a mouse model. J Orthop Res. 2002;206:1232–9.
-
Granero-Molto F, Weis JA, Miga MI, Landis B, Myers TJ, O'Rear L, Longobardi L, Jansen ED, Mortlock DP, Spagnoli A. Regenerative effects of transplanted mesenchymal stalk cells in fracture healing. Stem Cells (Dayton, Ohio). 2009;278:1887–98.
-
Shirley D, Marsh D, Hashemite kingdom of jordan G, McQuaid South, Li G. Systemic recruitment of osteoblastic cells in fracture healing. J Orthop Res. 2005;235:1013–21.
-
Taguchi Grand, Ogawa R, Migita M, Hanawa H, Ito H, Orimo H. The function of bone marrow-derived cells in bone fracture repair in a green fluorescent poly peptide chimeric mouse model. Biochem Biophys Res Commun. 2005;3311:31–6.
-
Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii Due north, Nagasawa T, Nakamura T. Stromal cell-derived gene 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;603:813–23.
-
Raheja LF, Genetos DC, Yellowley CE. Hypoxic osteocytes recruit human MSCs through an OPN/CD44-mediated pathway. Biochem Biophys Res Commun. 2008;3664:1061–6.
-
Zou C, Luo Q, Qin J, Shi Y, Yang L, Ju B, Song Thousand. Osteopontin promotes mesenchymal stalk cell migration and lessens jail cell stiffness via integrin beta1, FAK, and ERK pathways. Jail cell Biochem Biophys. 2013;653:455–62.
-
Gmel G, Bissery A, Gammeter R, Givel JC, Calmes JM, Yersin B, Daeppen JB. Alcohol-owing injuries in admissions to a swiss emergency room--an analysis of the link between book of drinking, drinking patterns, and preattendance drinking. Booze Clin Exp Res. 2006;303:501–9.
-
McFarlane Air-conditioning, Browne D, Bryant RA, O'Donnell M, Silove D, Creamer M, Horsley K. A longitudinal analysis of booze consumption and the risk of posttraumatic symptoms. J Affect Disord. 2009;118(1–3):166–72.
-
Stock SR. Microcomputer tomography: methodology and applications. Boca Raton: CRC Press; 2008.
-
Weitkamp T, Haas D, Wegrzynek D, Rack A. ANKAphase: software for unmarried-distance stage retrieval from inline 10-ray phase-contrast radiographs. J Synchrotron Radiat. 2011;18Pt 4:617–29.
-
Paganin D, Mayo SC, Gureyev TE, Miller PR, Wilkins SW. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J Microsc. 2002;206Pt 1:33–40.
-
Doube 1000, Klosowski MM, Arganda-Carreras I, Cordelieres FP, Dougherty RP, Jackson JS, Schmid B, Hutchinson JR, Shefelbine SJ. BoneJ: free and extensible bone image analysis in ImageJ. Bone. 2010;476:1076–9.
-
O'Neill KR, Stutz CM, Mignemi NA, Burns MC, Murry MR, Nyman JS, Schoenecker JG. Micro-computed tomography cess of the progression of fracture healing in mice. Bone. 2012;506:1357–67.
-
Obermeyer TS, Yonick D, Lauing M, et al. Mesenchymal stem cells facilitate fracture repair in an booze-induced impaired healing model. J Orthop Trauma. 2012;26(12):712–8.
-
Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stalk cells from mouse bone marrow. Nat Protoc. 2009;iv(1):102–6.
-
Franzin C, Piccoli 1000, Urbani C, Biz C, Gamba P, De Coppi P, Pozzobon M. In: Turksen K, editor. Isolation and expansion of musculus precursor cells from human being skeletal muscle biopsies, Stalk cell heterogeneity: methods and protocols; 2016. p. 195–204.
-
Roper PM, Abbasnia P, Vuchkovska A, Natoli RM, Callaci JJ. Alcohol-related scarce fracture healing is associated with activation of FoxO transcription factors in mice. J Orthop Res. 2016;3412:2106–xv.
-
Zou C, Song G, Luo Q, Yuan L, Yang 50. Mesenchymal stem cells require integrin beta1 for directed migration induced by osteopontin in vitro. In vitro cellular and developmental biology. Brute. 2011;473:241–50.
-
Sampson HW, Chaput CD, Brannen J, Probe RA, Guleria RS, Pan J, Baker KM, VanBuren V. Booze induced epigenetic perturbations during the inflammatory phase of fracture healing. Exp Biol Med (Maywood, NJ). 2011;23612:1389–401.
-
Hiltunen A, Vuorio Eastward, Aro HT. A standardized experimental fracture in the mouse tibia. J Orthop Res. 1993;112:305–12.
-
Holstein JH, Garcia P, Histing T, Kristen A, Scheuer C, Menger Medico, Pohlemann T. Advances in the establishment of defined mouse models for the study of fracture healing and os regeneration. J Orthop Trauma. 2009;235(Suppl):S31–8.
-
Blum A, Zarqh O, Peleg A, Sirchan R, Blum Due north, Salameh Y, Ganaem M. Vascular inflammation and endothelial dysfunction in fracture healing. Am J Orthop (Belle Mead, NJ). 2012;412:87–91.
-
Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE. Impaired angiogenesis, early callus germination, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res. 2007;222:286–97.
-
Hunter GK. Role of osteopontin in modulation of hydroxyapatite formation. Calcif Tissue Int. 2013;934:348–54.
-
Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;47:e249.
-
Colnot C, Zhang Ten, Knothe Tate ML. Electric current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous technology approaches. J Orthop Res. 2012;3012:1869–78.
-
Chen Q, Shou P, Zhang Fifty, Xu C, Zheng C, Han Y, Li W, Huang Y, Zhang Ten, Shao C, Roberts AI, Rabson AB, Ren Chiliad, Zhang Y, Wang Y, Denhardt DT, Shi Y. An osteopontin-integrin interaction plays a critical role in directing adipogenesis and osteogenesis past mesenchymal stem cells. Stem Cells (Dayton, Ohio). 2014;322:327–37.
-
Yamazaki M, Nakajima F, Ogasawara A, Moriya H, Majeska RJ, Einhorn TA. Spatial and temporal distribution of CD44 and osteopontin in fracture callus. J Os Joint Surg Br. 1999;813:508–15.
-
Stegen S, van Gastel Northward, Carmeliet G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone. 2015;70:xix–27.
-
Holstein JH, Becker SC, Fiedler Grand, Scheuer C, Garcia P, Histing T, Klein One thousand, Pohlemann T, Menger Dr.. Practice enhances angiogenesis during os defect healing in mice. J Orthop Res. 2011;297:1086–92.
-
Ling L, Nurcombe V, Absurd SM. Wnt signaling controls the fate of mesenchymal stalk cells. Gene. 2009;433(1–2):i–vii.
Acknowledgements
This enquiry was supported by funding from (one) NIH/NIAAA (AA021225, AA025551) to JJC, (2) OREF Clinician Scientist Award (12-024) to RMN, and (3) AONA (Kathryn Cramer Memorial Award) to MCM.
Funding
This research was supported past funding from the post-obit:
-
NIH/NIAAA (AA021225, AA025551) to JJC
-
OREF Clinician Scientist Laurels (12–024) to RMN
-
AONA (Kathryn Cramer Memorial Honour) to MCM.
Availability of information and materials
Delight contact writer for data requests.
Author information
Affiliations
Contributions
RMN received grant funding to back up the projection, is the primary writer of the manuscript, and fabricated contributions to all aspects of the experimental. HY was involved in data collection for both the μCT and in vitro MSC migration experiments. MCMM received grant funding to support the projection and was involved in both the preliminary studies the manuscript is based on and the SDF1α experiment data acquisition and analysis. PR oversaw about of the actual experimental procedures and data analysis presented in the manuscript, and was involved in the editing of the manuscript. PA and AV conducted the in vitro migration experiments. 20 and SRS were technical consultants for the μCT-based experiments. JJC is the Principal Investigator, in whose laboratory the studies were conducted. JJC received grant funding to support the studies, was involved in all aspects of the project, and co-wrote the manuscript with RMN. All authors read and approved the terminal manuscript.
Corresponding author
Ethics declarations
Ideals approval and consent to participate
Our laboratory received approval to deport the animal studies described in this manuscript from the Loyola University Institutional Animate being Care and Employ Commission (IACUC). Letter of approval is on file with JOSR.
Competing interests
The authors declare that they have no competing interests.
Publisher's Notation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/past/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Artistic Commons license, and indicate if changes were made. The Artistic Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/i.0/) applies to the information fabricated bachelor in this article, unless otherwise stated.
Reprints and Permissions
Most this article
Cite this article
Natoli, R.M., Yu, H., Meislin, M.CM. et al. Alcohol exposure decreases osteopontin expression during fracture healing and osteopontin-mediated mesenchymal stem cell migration in vitro. J Orthop Surg Res 13, 101 (2018). https://doi.org/10.1186/s13018-018-0800-7
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s13018-018-0800-7
Keywords
- Bone fracture
- Fracture not-marriage
- Booze
- Osteopontin
- Integrin
- Mesenchymal stem prison cell migration
Source: https://josr-online.biomedcentral.com/articles/10.1186/s13018-018-0800-7
Post a Comment for "When Can I Drink Again After Breaking My Bone"